Does Acetone React with Stainless Steel? A Comprehensive Guide
The question of whether acetone reacts with stainless steel is a common one, especially in industries and applications where both materials are frequently used. Acetone, a powerful solvent, is widely employed for cleaning, degreasing, and dissolving various substances. Stainless steel, known for its corrosion resistance and durability, is a staple in numerous sectors, from manufacturing to healthcare. Understanding their interaction is crucial for maintaining equipment integrity and ensuring safety.
This article delves into the compatibility of acetone and stainless steel, exploring their chemical properties, potential reactions, and practical implications. We’ll examine scientific evidence, industry best practices, and real-world scenarios to provide a comprehensive understanding of this important topic.
Understanding Acetone and Stainless Steel
To assess whether acetone reacts with stainless steel, it’s essential to understand the characteristics of each material.
Acetone: A Powerful Solvent
Acetone (CH3COCH3), also known as propanone, is a colorless, volatile, and flammable liquid. It’s a highly effective solvent, capable of dissolving a wide range of organic compounds, including fats, oils, waxes, and resins. Acetone is commonly used in nail polish remover, paint thinner, and industrial cleaning agents. Its chemical structure allows it to break down the intermolecular forces holding many substances together, making it an excellent solvent.
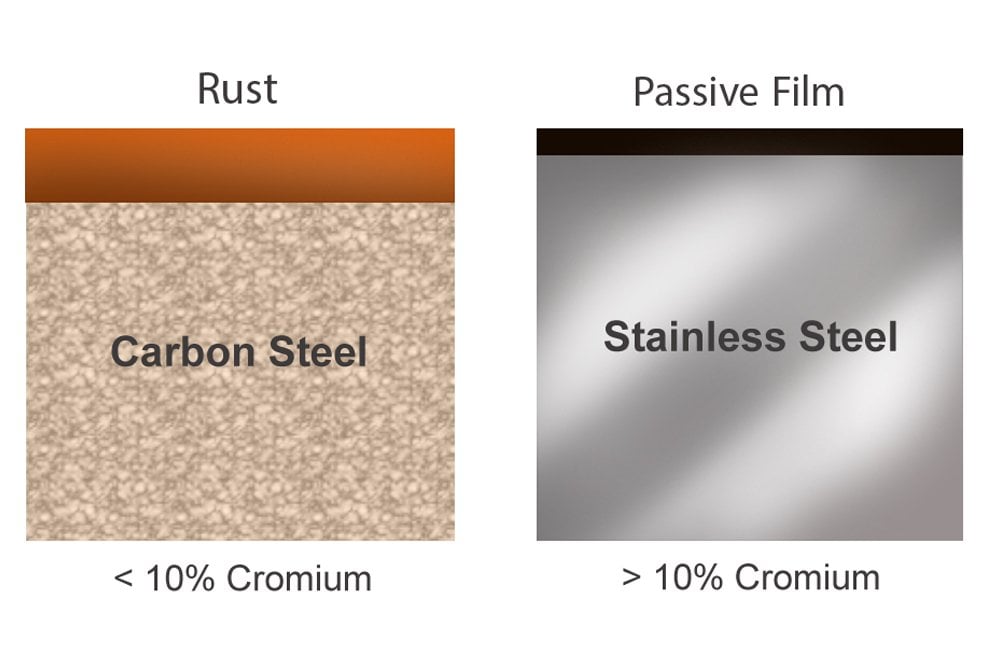
Stainless Steel: The Corrosion-Resistant Alloy
Stainless steel is an alloy of iron, chromium, and other elements, such as nickel, molybdenum, and titanium. The presence of chromium (typically at least 10.5%) forms a passive layer of chromium oxide on the surface, which protects the underlying steel from corrosion. Different grades of stainless steel offer varying levels of corrosion resistance and mechanical properties. Common grades include 304, 316, and 430, each with specific compositions and applications.
The Interaction Between Acetone and Stainless Steel
So, does acetone react with stainless steel? In most cases, the answer is no. Acetone is generally considered compatible with stainless steel. However, several factors can influence their interaction.
Chemical Compatibility
Acetone is a relatively inert solvent when it comes to stainless steel. It does not typically corrode or degrade stainless steel under normal conditions. The passive chromium oxide layer on stainless steel provides a robust barrier against chemical attack. Acetone doesn’t react with this layer in a way that would compromise its protective function.
Concentration and Exposure Time
The concentration of acetone and the duration of exposure can play a role. While pure acetone is unlikely to damage stainless steel, prolonged exposure to high concentrations, especially at elevated temperatures, might have some effect. The risk is generally low, but it’s worth considering in specific applications. Regularly wiping down surfaces after acetone exposure can further mitigate any potential concerns.
Presence of Contaminants
The presence of contaminants in the acetone can significantly alter its interaction with stainless steel. For example, if the acetone contains water and chlorides, it can increase the risk of corrosion. Chlorides are known to attack the passive layer on stainless steel, leading to pitting corrosion. Therefore, using high-quality, pure acetone is recommended when working with stainless steel.
Grade of Stainless Steel
The grade of stainless steel also matters. Some grades are more resistant to chemical attack than others. For instance, 316 stainless steel, which contains molybdenum, offers better corrosion resistance than 304 stainless steel. If you’re using acetone frequently, especially in harsh conditions, choosing a higher grade of stainless steel can provide an extra layer of protection. [See also: Stainless Steel Grades Comparison]
Practical Considerations and Best Practices
While acetone is generally safe to use with stainless steel, following certain best practices can help ensure long-term compatibility and prevent any potential issues.
Proper Ventilation
Acetone is a volatile organic compound (VOC), and its vapors can be harmful if inhaled in high concentrations. Always work in a well-ventilated area when using acetone. This helps to minimize exposure to the vapors and reduces the risk of respiratory irritation or other health effects.
Personal Protective Equipment (PPE)
Wear appropriate personal protective equipment (PPE) when handling acetone. This includes gloves, eye protection, and, if necessary, a respirator. Gloves protect your skin from direct contact with acetone, which can cause dryness and irritation. Eye protection, such as safety glasses or goggles, prevents acetone from splashing into your eyes. A respirator may be needed if you’re working in an area with poor ventilation or high acetone concentrations.
Cleaning and Maintenance
Regularly clean and maintain stainless steel equipment that comes into contact with acetone. Wipe down surfaces after each use to remove any residual acetone. This prevents prolonged exposure and reduces the risk of any potential damage. Also, inspect the stainless steel for signs of corrosion or degradation and address any issues promptly.
Material Compatibility Testing
In critical applications, such as those in the aerospace or pharmaceutical industries, it’s advisable to conduct material compatibility testing. This involves exposing stainless steel samples to acetone under controlled conditions and monitoring for any signs of corrosion or degradation. This testing can provide valuable data to ensure the long-term reliability of your equipment. [See also: Material Compatibility Testing Methods]
Real-World Applications and Examples
To further illustrate the interaction between acetone and stainless steel, let’s consider some real-world applications and examples.
Industrial Cleaning
In manufacturing and industrial settings, acetone is often used to clean stainless steel equipment and parts. For example, it can remove grease, oil, and other contaminants from stainless steel machinery. Because acetone generally does not react with stainless steel, it’s a popular choice for this purpose, offering effective cleaning without damaging the equipment.
Laboratory Settings
In laboratories, acetone is used to clean glassware and other lab equipment made of stainless steel. The non-reactive nature of acetone with stainless steel makes it a safe and effective cleaning agent in these environments.
Aerospace Industry
The aerospace industry relies heavily on stainless steel components. Acetone is often used for pre-painting cleaning and surface preparation. Its ability to remove contaminants without compromising the integrity of stainless steel makes it a valuable tool in this industry. However, stringent quality control measures are in place, including material compatibility testing, to ensure the safety and reliability of aerospace components. [See also: Aerospace Material Standards]
Potential Problems and Solutions
While acetone is generally considered safe for use with stainless steel, there are certain situations where problems can arise. Understanding these potential issues and their solutions is crucial for preventing damage and ensuring the longevity of your equipment.
Pitting Corrosion
As mentioned earlier, the presence of chlorides in acetone can lead to pitting corrosion in stainless steel. This is particularly true if the stainless steel is exposed to acetone for extended periods. To prevent pitting corrosion, use high-purity acetone and avoid contamination with chlorides. Regularly clean and inspect the stainless steel for signs of pitting.
Crevice Corrosion
Crevice corrosion can occur in areas where there are small gaps or crevices in the stainless steel. These areas can trap acetone and any contaminants, leading to localized corrosion. To prevent crevice corrosion, design equipment to minimize crevices and ensure proper sealing. Regularly clean and inspect crevices for signs of corrosion.
Stress Corrosion Cracking
Stress corrosion cracking (SCC) can occur when stainless steel is subjected to tensile stress in the presence of a corrosive environment. While acetone itself is unlikely to cause SCC, contaminants such as chlorides can exacerbate the problem. To prevent SCC, minimize tensile stress in stainless steel components and use high-purity acetone. Consider using a more corrosion-resistant grade of stainless steel if SCC is a concern.
Conclusion
In conclusion, acetone generally does not react with stainless steel under normal conditions. Stainless steel’s inherent corrosion resistance, due to its passive chromium oxide layer, makes it compatible with acetone for many applications. However, factors such as the concentration of acetone, the presence of contaminants, and the grade of stainless steel can influence their interaction. By following best practices, such as using high-purity acetone, ensuring proper ventilation, and regularly cleaning and inspecting equipment, you can safely use acetone with stainless steel and maintain the integrity of your equipment.
Understanding the compatibility of acetone and stainless steel is essential for various industries, from manufacturing to laboratories. By considering the factors discussed in this article, you can make informed decisions and ensure the safe and effective use of both materials.
